腫瘤的生長和代謝可能會局限於某些氨基酸用於蛋白合成。最近已經被證明,某些類型的癌細胞依賴於甘氨酸、穀氨酰胺,亮氨酸,絲氨酸代謝和增殖。此外,運用左旋門冬酰胺酶誘導天冬酰胺缺乏,已經用於治療急性淋巴細胞白血病。然而,本研究之前還不能在每個腫瘤中檢測哪些氨基酸缺乏可以導致其生長受阻。
在2月25日的Nature中,研究者用核糖體分析來檢測限製性氨基酸。他們開發了diricore,一種核糖體檢測不同密碼子的步驟,用來評估特定氨基酸用於蛋白合成的可用性。
Diricore是基於核糖體分析檢測(ribosome profiling)而開發的,核糖體分析是一種基於深度測序的技術可以定量地分析核苷酸翻譯的方法。使用核酸酶消化mRNA時,在翻譯過程中發揮作用的核糖體結合並保護了大約30bp的mRNA片段(RPF)。將細胞中這些被保護的mRNA片段構建成DNA文庫,再使用測序儀對文庫中所有的片段進行深度測序,最終得到了有關細胞中蛋白質翻譯情況的圖譜。而在此基礎上如圖,diricore運用RPF進行亞克隆測序和5’末端密度兩種互補的方法進行分析。
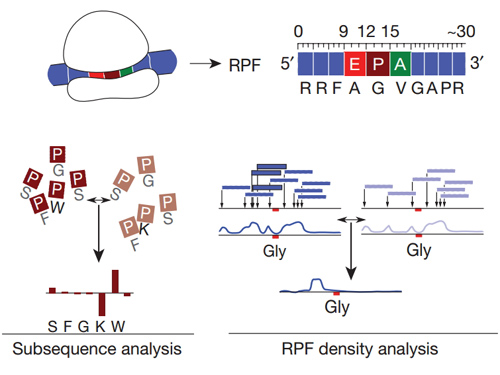
RPF的9,12和15位點分別對應核糖體的E,P和A位點
他們首先運用代謝抑製和營養缺乏分析驗證了diricore的功能與抑製。值得注意的是使用左旋門冬酰胺酶在天冬酰胺密碼子誘導特殊diricore信號會引起高水平天冬酰胺合成酶(ASNS)。
然後研究者將diricore運用到腎癌上,從信號上發現脯氨酸是限製性的氨基酸。對於天冬酰胺來說,觀察的結果與高水平的PYCR1,脯氨酸產生的一個關鍵酶相關。這提示了一個允許腫瘤擴增的代償性的機製。PYCR1是由脯氨酸前體不足引起的,當脯氨酸缺乏時抑製該酶可以抑製腎癌細胞的增殖。
高水平的PYCR1經常在浸潤性的乳腺癌中被觀察到。在這個腫瘤的體內模型係統中,研究人員也發現了脯氨酸是限製性氨基酸。在限製生長的條件下,需要PYRC1來維持腫瘤生長。進一步研究表明,CRISPR介導的PYCR1的基因敲除可以阻礙這個模型係統中腫瘤的生長。
因此diricore可以用於揭示哪些對於癌細胞來說是限製性的氨基酸,尋找哪些能作為癌症治療的重要代謝途徑的靶點。
Tumour growth and metabolic adaptation may restrict the availability of certain amino acids for protein synthesis. It has recently been shown that certain types of cancer cells depend on glycine, glutamine, leucine and serine metabolism to proliferate and survive. In addition, successful therapies using L-asparaginase-induced asparagine deprivation have been developed for acute lymphoblastic leukaemia. However, a tailored detection system for measuring restrictive amino acids in each tumour is currently not available. Here we harness ribosome profiling6 for sensing restrictive amino acids, and develop diricore, a procedure for differential ribosome measurements of codon reading. We first demonstrate the functionality and constraints of diricore using metabolic inhibitors and nutrient deprivation assays. Notably, treatment with L-asparaginase elicited both specific diricore signals at asparagine codons and high levels of asparagine synthetase (ASNS). We then applied diricore to kidney cancer and discover signals indicating restrictive proline. As for asparagine, this observation was linked to high levels of PYCR1, a key enzyme in proline production, suggesting a compensatory mechanism allowing tumour expansion. Indeed, PYCR1 is induced by shortage of proline precursors, and its suppression attenuated kidney cancer cell proliferation when proline was limiting. High PYCR1 is frequently observed in invasive breast carcinoma. In an in vivo model system of this tumour, we also uncover signals indicating restrictive proline. We further show that CRISPR-mediated knockout of PYCR1 impedes tumorigenic growth in this system. Thus, diricore has the potential to reveal unknown amino acid deficiencies, vulnerabilities that can be used to target key metabolic pathways for cancer treatment.